Besler et al.: Determination
of Hidden Allergens in Foods by Immunoassays
Internet Symposium on Food Allergens
4(1): 1-18 (2001) [http://www.food-allergens.de]
INTRODUCTION
The problem of hidden food allergens has been recognized for decades
(Miller 1978). In most cases hidden food allergens may induce only mild
symptoms in allergic subjects, but tragically even fatal events have occurred
after inadvertent ingestion (Sampson 1998, Wüthrich 2000, Bock 2001).
In Canada and the USA the food authorities frequently publish alerts on
recalls of food products which may contain most severe food allergens not
declared on the labels. Labeling of food products for the presence of food
allergens is at present the most effective way to enable food allergic
individuals to avoid the ingestion of hidden allergens. Therefore the aim
of allergen determination in foods is of major concern for both the food
industry and the food allergic consumer, and testing foods for the presence
of allergens should have a definite place in the HACCP (hazard analysis
and critical control point) plans and allergen control plans of food manufacturers
(Deibel et al. 1997, Hugget & Hischenhuber 1998).
Only recently the FAO/WHO and the European Commission proposed a list
of allergens which have to be labelled on prepackaged foods regardless
of the amounts present. The allergen lists are based on the prevalence
and severity of the related allergies. The stability of these food allergens,
their allergenic potential and frequency in processed foods should be considered
as well (Bousquet et al. 1998, Yeung et al. 2000). The Codex Alimentarius
standard includes milk, eggs, fish, crustaceae, peanut, soybean, tree nuts,
and wheat (gluten-containing cereals), while the European proposal additionally
includes sesame seeds (Table 1). The food allergens to be included should
be subject to a continuing scientific evaluation. For example celery is
not included although the scientific criteria for inclusion have been fulfilled
recently (Ballmer-Weber et al. 2000). Currently both sets of labeling regulations
do not cover allergen contamination of food products by "cross-contact".
US-Attorneys called for reforms in food labelling and processing in
a recent Citizen Petition to the U.S. Food and Drug Administration (2000).
The petition demanded a symbol on the label to alert consumers that the
product in the package contains allergens such as peanuts, tree nuts, milk,
eggs, fish, crustaceans, molluscs, wheat or soybeans; declaration when
allergenic ingredients are used even in small amounts that are currently
designated as "insignificant levels"; a toll-free hotline where consumers
can obtain reliable food ingredient information, and food industry guidelines
to prevent the migration of allergenic ingredients from one product to
another during food processing and preparation.
For these reasons the detection and determination of hidden allergens
in foods is becoming more and more important. There is clearly a need for
analytical methods which are highly specific and sensitive in detecting
even trace amounts of allergens. These methods need to be rapid, robust,
reliable, and cost-effective. This review gives a short overview of circumstances
leading to the presence of hidden allergens in foods. After discussing
the amounts of hidden allergens in foods which can elicit allergic symptoms,
the analytical methods for the detection of food allergens are introduced
in detail. A brief explanation of the principle of each detection method
is followed by some selected applications. It should be noted that the
cited methods have been selected on the basis of sufficient limits of detection
and successful application to authentic food samples (for a recent review
including a broader range of applications see Besler 2001). Assays for
the determination of wheat proteins (gluten / gliadins) are not included.
These methods were recently reviewed by Denery-Papini et al. (1999).
Table 1: List of food allergens to be labelled on prepackaged foods
FAO/WHO Standard
(Codex Alimentarius Commission 1999) |
Amendment of Labelling Directive 2000/13/EC
(Proposal from the European Commission 2001) |
| Milk |
Milk |
| Hen's Egg |
Hen's Egg |
| Fish |
Fish |
| Crustaceae |
Crustaceae |
| Peanut |
Peanut |
| Tree Nuts |
Tree Nuts |
| Soybean |
Soybean |
| Wheat |
Wheat |
| |
Sesame Seed |
SOURCES
OF HIDDEN ALLERGENS IN FOOD PRODUCTS
Circumstances of
food manufacture which result in the presence of hidden allergens in foods
include many potential sources (Deibel et al. 1997, Hugget &
Hischenhuber 1998). Global trade and transport often makes it extremely
difficult to exclude the presence of certain allergenic compounds. Major
reasons for the occurrence of hidden allergens in processed foods are:
-
Cross-contact, which is a problem arising from using the same equipment
for the production of foods containing a specific allergenic compound and
for the production of foods not containing this compound (shared equipment).
-
Carry-over of an allergenic compound may occur during food production,
for example if inappropriate rework containing an allergenic ingredient
is used.
-
Changes of the formulation of a product without appropriate changes
on the label.
-
Incomplete or incorrect lists of ingredients
-
The raw materials may contain unknown ingredients
-
Misinterpretation of common names or ingredients could be derived
from allergenic sources which are not indicated on the label
-
Exemptions of labelling in the labelling regulations. For example
ingredients of a compound which constitutes less than 25% of the food product
do not have to be labelled. (The so-called 25%-rule will be deleted according
to the proposed amendment of the EU labelling directive.)
AMOUNTS
AND THRESHOLDS OF HIDDEN FOOD ALLERGENS
Only in a minority of allergic events involving the ingestion of food
products did it prove possible to quantitate or even identify the allergenic
source. Some cases where the allergenic source was determined are given
in Table 2. The detected food allergens include peanut, hazelnut, milk,
and egg. Ingested foods were a dry soup, chocolate, cookies, a fruit sorbet,
icecream, a sausage and pasta. Generally the ingested amount of protein
ranged from 10 to 100 mg. In only two cases was the ingestion of lower
amounts described. The first case involved the hidden presence of hazelnut
protein in a chocolate. 700 µg of hazelnut protein were reportedly
ingested. The other event occurred after ingestion of 120-180 µg
of whey proteins in a fruit sorbet. On the basis of ingestion of 100 g
of a respective food the lowest concentrations of hidden allergens were
about 1.2-1.8 mg/kg and 7 mg/kg, while the concentrations ranged from 100
to 1000 mg/kg in the other reports.
Taylor et al. (2002) identified considerable data related to the threshold
doses for peanut, cow's milk, and egg, analyzing clinical files; only limited
data were available for other foods, such as fish and mustard. However,
the authors concluded that the estimation of a threshold dose is very difficult
and a standardized protocol for clinical experiments to allow determination
of the threshold dose should be developed.
The lowest doses eliciting allergic symptoms in DBPCFC studies were
4 mg of peanut, 6 mg of codfish, and 50 mg of egg white (Hourihane
et al. 1997, Hansen & Bindslev-Jensen 1992, Norgaard & Bindslev-Jensen
1992). Short-lived, subjective symptoms occurred after ingestion of 100
µg peanut protein. While severe, systemic reactions were induced
by ingestion of 5 mg peanut protein (Hourihane et al. 1997). Assuming an
ingestion of 100 g of an offending food, a concentration of at least 50
mg/kg peanut protein should be detectable in processed foods with respect
to severe allergic reactions.
Most recently Morisset & Moneret-Vautrin (2001) proposed threshold
levels of clinical reactivity to food allergens evaluating a standardized
placebo-controlled oral challenge protocol. In this study cases of severe
food allergy corresponded to positive oral challenges with cumulative reactive
doses of less than 6.5 mg of egg protein, 32 mg of milk protein, 16 mg
of peanut protein, and 12 mg of sesame protein. On the basis of an ingestion
of 100 g of an offending food the authors demand assay detection limits
of 65 mg/kg for egg proteins, 300 mg/kg for milk proteins, and 165 mg for
peanut proteins in foods. However 0.8% of 125 egg allergic patients, 1.7%
of 59 milk allergic patients, and 3.9% of peanut allergic patients reacted
to even lower cumulative doses. For these patients the assays should be
more sensitive (10 mg/kg for egg protein, 30 mg/kg for milk protein, and
24 mg/kg for peanut protein, respectively).
Table 2: Ingested Amounts of Hidden Allergens Reportedly Eliciting
Allergic Symptoms
| Hidden Allergen |
Amount of Protein |
Ingested Food |
Reference |
| Peanut |
45 mg |
Dry Soup |
McKenna & Klontz 1997 |
| Hazelnut |
700 µg (Corylin) |
Chocolate |
European Commission 1998 |
| Hazelnut |
50 mg (Corylin) |
Cookies |
European Commission 1998 |
| Milk |
120-180 µg (Whey Proteins) |
Fruit Sorbet |
Laoprasert et al. 1998 |
| Milk |
60 mg (Caseins) |
Sausage |
Malmheden Yman et al. 1994 |
| Milk |
10 mg (Caseins) |
Soy-based Icecream |
European Commission 1998 |
| Hen's Egg |
10 mg (Ovalbumin) |
Pasta |
European Commission 1998 |
| Hen's Egg |
100 mg (Ovalbumin) |
Cookies |
European Commission 1998 |
ANALYTICAL
METHODS FOR THE DETECTION OF FOOD ALLERGENS
Nearly all food allergens
are proteins or glycoproteins with a molecular mass ranging from about
10 to 70 kDa. Immunological methods have been applied for the characterization
of food allergens since they were first identified. The most common methods
for the detection of food allergens are summarized in Table 3. Immunoassays
involving human IgE antibodies are mainly used to characterize the allergenic
properties of a protein, while immunoassays using animal antisera detect
certain proteins used for the immunization of the animal during antibody
production, but not specifically an "allergenic protein" or "allergen".
The detection of allergens by human IgE-antibodies include radio-allergosorbent
test (RAST) inhibition or enzyme-allergosorbent test (EAST) inhibition
methods. These methods are variations of the RAST or EAST applications
usually used for the characterization of patient's sera determining specific
IgE-levels. SDS-PAGE immunoblot techniques can be used for the identification
and characterization of major and minor food allergens. Although specific
IgE is required for allergen characterization it is not suitable for reliable
allergen determination in food products, since the specificity of IgE from
sensitized individuals differs considerably and the amount of sera is usually
limited. Moreover, multiple sensitivities and/or cross-reactivities to
more than one allergenic food may be present in human serum-IgE.
Detection methods involving antibodies from rabbits, mice, goats, sheep,
or chicken include immunodiffusion techniques, rocket-immunoelectrophoresis,
dot-immunoblotting, SDS-PAGE immunoblotting, and enzyme-linked immunosorbent
assays (ELISA-Techniques). With the exception of immunodiffusion techniques,
which are not sensitive enough, these methods are used for the detection
and in some cases for the quantitation of food allergens. The ELISA techniques
are the most promising tools for the determination of hidden allergens
in foods.
Detecting DNA from allergenic sources is just at the beginning of its
development. Only very few applications of PCR-reactions for the detection
of allergens, namely hazelnut and wheat, have been published (Koeppel et
al. 1998, Holzhauser et al. 2000). PCR methods are not further discussed
here (for a brief discussion of PCR-based methods see Besler 2001).
Table 3: Analytical methods for the detection of food allergens
| Detection of Allergen |
Detection of Protein |
Detection of DNA |
-
Immunoassays involving Human IgE Antibodies
|
-
Immunoassays involving Antibodies from Rabbits, Mice, Goats,
Sheep, or Chicken
|
-
Encoding for a Specific Protein
|
-
RAST / EAST-Inhibition
-
SDS-PAGE / Immunoblotting
|
-
Immunodiffusion
-
Rocket-Immunoelectrophoresis
-
Dot-Immunoblotting
-
SDS-PAGE / Immunoblotting
-
ELISA
|
|
COMMON
CRITERIA FOR IMMUNOASSAYS
Some general recommendations must be considered in performing immunoassays.
The sample preparation is always a most critical step. An analytical method
can only be as good as the sample preparation is. An important characteristic
is the extraction efficiency, depending on the food matrix to be analysed.
Acceptable recoveries for ELISA methods vary between 70 and 120% with coefficients
of variation (CV) of less than 20% (Lipton et al. 2000).
The sensitivity and limits of detection and quantitation, respectively,
should meet the requirements of detecting even trace amounts of allergens
in foods. As mentioned above, detecting amounts as low as 1-100 mg/kg are
required as limits of detection for some food allergens. Furthermore an
immunoassay should be specific. Therefore cross-reactivities should be
excluded or well-characterized, respectively. The antibody specificity
depends, for example on the purity of the used immunogen (e.g. crude protein
extract or purified protein) and its similarity to other proteins. Therefore
antibody specificity must be tested. In order to minimize cross-reactivities
antisera can be preabsorbed with related food items. For example anti-hazelnut
corylin antibodies preabsorbed against various nuts and anti-peanut antibodies
preabsorbed against soybean, white bean, and marzipan (almonds) are commercially
available (Holzhauser et al. 1999a, 1999b). Moreover, antisera must be
capable of detecting allergens in processed foods. Thus antibodies raised
with native food protein extracts may not be or may be less reactive to
food proteins denatured by various treatments during food processing. This
can be circumvented by raising antibodies with protein extracts from pre-treated
foods such as roasted peanuts or hazelnuts.
The need of a thorough quality control even when a commercial test kit
is used is demonstrated by Keck-Gassenmeier et al. (1999), who employed
a commercial ELISA test kit for the determination of peanut protein in
dark chocolate. They showed that the extraction method supplied by the
test kit manufacturer was not sufficient to detect trace amounts of peanut
protein in dark chocolate. By the simple addition of 10% fish gelatine
to the extraction buffer the recovery rates improved from 2-3% to 63-89%
for amounts as low as 2 mg/kg. The authors attributed the striking improvement
of the recoveries to tannin-binding properties of fish gelatine. Interestingly
the investigation of milk chocolate revealed no difference for both extraction
buffers (with and without fish gelatine) which was probably due to the
higher amount of milk proteins and lower amount of cacao (tannin). Furthermore
the different results of spiking dark chocolate with peanut proteins or
peanut butter underlined the importance of analysing recoveries under almost
real-life conditions.
Similarly the limits of detection may differ for different food matrices.
Blais & Phillipe (2001) demonstrated a 10 fold variation of the limit
of detection of hazelnut protein investigating nine different foods. In
this study the lowest limit of detection was found for a cake mix (0.12
mg/kg), while the highest detection limits were found for almond and fruit
bars (both 1 mg/kg).
Antibody-Containing Gel
Antigen-Antibody Precipitation
Antigen Standards |
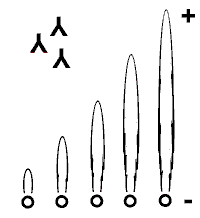 |
Figure 1: Principle of rocket-immunoelectrophoresis |
ROCKET
IMMUNOELECTROPHORESIS
Principle
Rocket-immunoelectrophoresis employs an antibody-containing gel (Figure
1). The standard or sample proteins (antigens) migrate according to their
electrophoretic mobility until antigen-antibody-complexes precipitate in
the gel. Rocket-shaped precipitates are build at a constant antigen / antibody
ratio. The height of the rockets is proportional to the amount of antigen
applied. |
Applications
The presence of undeclared allergens was detected by rocket-immunoelectrophoresis
in various food products (Table 4). Egg, hazelnut, milk and peanut proteins
could be analyzed with a detection limit of 30 mg/kg. The sensitivity or
range of detection was 25-420 µg/mL using Coomassie brilliant blue
for staining of gels (Malmheden Yman et al. 1994).
A more sensitive application was described by Holzhauser & Vieths
(1998). The detection of peanut proteins was improved by a staining method
involving an enzyme-labeled anti-rabbit IgG antibody. The sensitivity ranged
from 20 to 1440 ng/mL, resulting in a superior limit of detection of 2.5
mg/kg.
Major disadvantages of rocket-immunoelectrophoretic applications are
the rather uneasy and time consuming handling of gel preparation and immunostaining
procedures.
Table 4: Applications of rocket-immunoelectrophoresis for the detection
of food allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
a) Egg (Ovalbumin)
b) Hazelnut (Corylin)
c) Milk (Caseins)
d) Peanut (Protein)
Sensitivity:
25-420 µg/mL |
not available
Antisera:
rabbit Ab (a, b, c), sheep Ab (d) |
Samples:
a) Meat Balls, Pasta
b) Chocolate
c) Ice Cream, Chocolate, Lollipop, Sausage, Hot Dog,
Recombined Ham, Meringue
d) Cake
Limit of Detection:
30 mg/kg |
Malmheden Yman et al. 1994 |
| Peanut (Protein)
Sensitivity:
20-1440 ng/mL
(Peanut Protein) |
No (20 Legumes, Nuts, and other Ingredients tested)
Antiserum (in Gel):
rabbit Ab |
Samples: Candy, Chocolate Products, Cornflakes,
Ice Cream, Muesli, Rice Cracker
Limit of Quantitation:
2.5 mg/kg
Recovery: 85-101%
CV: <5% |
Holzhauser & Vieths 1998 |
| Membrane Strips
Antigen Standards
Antibody
(Enzyme-labelled)
Substrate
Product |
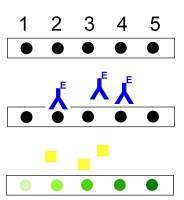 |
Figure 2: Principle of dot-immunoblotting |
Principle
In dot-immunoblotting the standards and samples are spotted onto membrane
strips. Specific detection is achieved by incubation with enzyme-labeled
antibodies which bind to the target antigens. The spots are visualized
by addition of a substrate which is transformed by an enzymic reaction
into a colored product. The intensity of the spots is proportional to the
amount of antigen. |
Applications
Recently a dot-immunoblotting application was described for the detection
of peanut proteins in various foods (Blais & Phillipe 2000). This method
is capable of detecting amounts as low as 2.5 mg/kg. Despite the fact that
no quantitation was performed, the method allows simple and inexpensive
screening of food samples.
Table 5: Applications of dot-immunoblotting for the detection of
food allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
| Peanut (Protein)
Sensitivity:
30 ng/mL
(Peanut Protein) |
No (Chick Pea, Lentils, Red Kidney Beans, Hazelnut, Brazil
Nut tested)
Antiserum:
chicken Ab (IgY) |
Samples:
Almond Butter, Bars, Chocolate Products, Cookies, Ice
Cream, Potato Chips
Limit of Detection:
2.5 mg/kg |
Blais & Phillippe 2000 |
Electrophoretic Separation
Membrane Strips
Antibody
(Enzyme-labelled)
Substrate
Product |
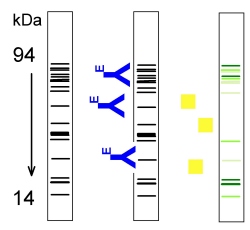 |
Figure 3: Principle of SDS/PAGE-immunoblotting |
Principle
Samples and standards are separated in SDS-Polyacrylamid-Gelelectrophoresis
according to their molecular mass. Afterwards the separated bands are transferred
onto a membrane and detected with enzyme-labeled antibodies as described
for dot-immunoblotting. This method allows the detection and identification
of individual proteins or allergens. |
Applications
Most recently an SDS-PAGE / immunoblot application for the qualitative
detection of almond and hazelnut proteins in chocolates was described by
Scheibe et al. (2001). The sensitivity of the method was about 200 ng/mL,
resulting in a limit of detection of 5 mg/kg. Schäppi et al. (2001)
detected the major peanut allergens (Ara h 1, 2, 3, and 4) in cereal bars,
corn crackers and potato snacks. The content of undeclared peanuts ranged
from 0.05 to 0.5% in the samples.
Table 6: Applications of SDS/PAGE-immunoblotting for the detection
of food allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
a) Almond
b) Hazelnut
Sensitivity:
200 ng/mL |
No (Hazelnut, Almond, Milk, Cocoa, Peanut)
Antisera:
rabbit pAb |
Samples:
Chocolates
Limit of Detection:
5 mg/kg |
Scheibe et al. 2001 |
| Peanut
Sensitivity:
- |
No IgE-binding cross-reactivity to other food allergens
Antisera:
human IgE |
Samples:
Cereal Bars, Corn Crackers, Potato Snack
Limit of Detection:
5-50 mg/kg |
Schäppi et al. 2001 |
| Product
Substrate
Antibody
(Enzyme-labelled)
Analyte
(Inhibitor)
Immobilized
Antigen
Solid Phase Support |
 |
|
|
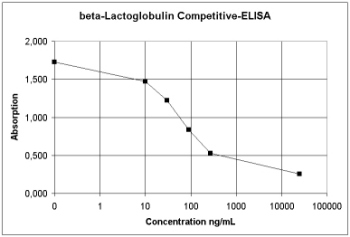
Figure 4: Principle of Competitive-ELISA |
Principle
Enzyme-linked Immunosorbent Assays are most frequently performed in
96-well microplates or in 8-well strips. The competitive ELISA involves
immobilized antigens bound to the solid phase. If no sample antigen is
present the enzyme-labelled antibody shows maximal binding to the solid
phase bound antigen, resulting in high absorption of the colored product
formed. Binding of the enzyme-labelled antibody is inhibited by increasing
amounts of antigen. The standard curve shows the typical sigmoid shape.
In this example the standard curve of beta-lactoglobulin, a whey protein,
is shown.
|
Applications
Applications of the Competitive-ELISA are shown in Table 7. The tests
for the detection of hazelnut and peanut proteins used polyclonal antisera
from rabbits, while the ELISA for the determination of beta-lactoglobulin
compared a polyclonal rabbit-antibody and a monoclonal mouse-antibody.
The hazelnut-ELISA was performed in the range of 5 to 1000 ng/mL with
a detection limit of 1 mg/kg (Koppelman et al. 1999). The recovery from
samples like chocolate, cookies, and cake ranged from 67 to 132%. Significant
cross-reactivities were observed for several nuts and peanuts. A similar
assay performance was described for the Peanut-ELISA by Holzhauser &
Vieths (1999a). Only a slightly poorer sensitivity and limit of detection
were observed.
A more sensitive Peanut-ELISA was described by Yeung & Collins
(1996). The sensitivity was between 1 and 63 ng/mL, resulting in a detection
limit of 0.4 mg/kg. No cross-reactivities were observed to 22 tested legumes,
nuts, and other food ingredients.
Mariager et al. (1994) determined beta-lactoglobulin in cow's milk
and infant formulas comparing a polyclonal antibody with a monoclonal antibody.
The polyclonal antibody offered a 3 to 4 fold broader range of detection
and a 30 fold lower limit of detection.
Table 7: Applications of Competitive-ELISA for the detection of food
allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
| Hazelnut (Protein)
Sensitivity:
5-1000 ng/mL
|
Walnut, Cashew, Almond, Brazil Nut, Peanut, Pine Nut
Antiserum:
rabbit pAb |
Samples: Chocolate Products, Cookies, Cake, Milk
Flavour
Limit of Detection:
1 mg/kg
Recovery: 67-132% |
Koppelman et al. 1999 |
| Peanut (Protein)
Sensitivity:
1-63 ng/mL
|
No (22 Legumes, Nuts, and other Ingredients tested)
Antiserum:
rabbit pAb |
Samples: Chocolate Bars, Cookies, Ice Cream, Mixed
Nuts and Seeds, Pasta Sauces
Limit of Detection:
0.4 mg/kg
Recovery: 68-90%
CV: 2-22% |
Yeung & Collins 1996 |
| Peanut (Protein)
Sensitivity:
24-1000 ng/mL
|
Walnut, Pinto Bean
Antiserum:
rabbit pAb |
Samples: Cashew, Chocolate, Nut and Chocolate,
Raisin, Coconut Cookies, Amarettini, Cereal Bars
Limit of Detection:
2 mg/kg
Recovery: 84-126%
CV: <15% |
Holzhauser & Vieths 1999a |
| Cow's Milk (beta-Lactoglobulin)
Sensitivity:
a) 0.1-1000 ng/mL
b) 4-50 ng/mL |
not available
Antisera:
a) rabbit pAb (against heat treated beta-Lactoglobulin)
b) mouse IgG mAb |
Samples: Whole Milk, Infant Formulas (ready to
use)
Limit of Detection:
a) 0.08 µg/L
b) 3.2 µg/L
CV: <33% |
Mariager et al. 1994 |
| Product
Substrate
Second
Antibody
(Enzyme-labelled)
Analyte
(Antigen)
Capture
Antibody
Solid Phase Support |
 |
|
|
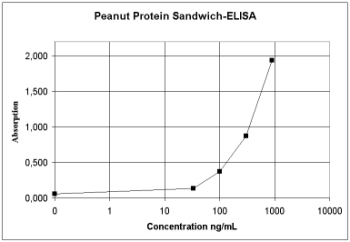
Figure 5: Principle of Sandwich-ELISA |
Principle
For the detection of proteins, sandwich ELISA is the most common type
of immunoassay performed. This format involves an immobilized capture antibody
on the microplate wells (Figure 5). After adding the standard or sample
solution antibody-analyte binding occurs. A second, analyte specific, labeled
antibody is added and also binds to the analyte, forming a "sandwich".
Then a substrate is added, reacting with the enzyme and producing a colored
product. The absorption is directly proportional to the concentration of
the analyte. The curve shows the peanut standards of a commercial ELISA-Test-Kit. |
Applications
Table 8 shows applications of Sandwich-ELISA. The Almond- and the Hazelnut-ELISA
involved rabbit and sheep polyclonal antisera as capture and secondary
antibodies, respectively, while the Peanut-ELISA used an unlabeled and
an enzyme-labeled rabbit polyclonal antiserum.
For determination of almonds a sensitivity of 100 ng/mL and a limit
of detection of 1 mg/kg was achieved. However, several seeds and nuts gave
significant cross-reactivities (Hlywka et al. 2000).
The sensitivity of the Hazelnut-ELISA ranged from 1 to 600 ng/mL, resulting
in a detection limit of 2 mg/kg (Holzhauser & Vieths 1999b). Tolerable
amounts of cross-reactive pumpkin seeds, walnut, and cashew (not interfering
with the detection of hazelnut protein) were determined. It seems very
useful to know the amounts of cross-reactive sample ingredients which can
be tolerated by the assay. So it can be estimated whether the test is applicable
for to a certain sample containing interfering ingredients or not.
The peanut application gave a detection limit of 0.1 mg/kg (Koppelman
et al. 1996). The sensitivity ranged from 5 to 1000 ng/mL. Cross-reactivities
were observed for almond and cashew. Tsuji et al. (1993, 1995) developed
a Sandwich-ELISA for the determination of the major soybean allergen (Gly
m Bd 30K). They used two monoclonal antibodies as capture and secondary
antibody, respectively. Within the range of 140-700 mg/kg, Gly m Bd 30K
was detected in various food products, while it was not detected in fermented
soybean products such as miso, shoyu, and natto.
Hefle et al. (2001) described a Sandwich-ELISA for the detection of
egg white in various pasta products. Interestingly the most sensitive ELISA-format
was achieved using a capture antibody raised against egg white and a detection
antibody specific for ovalbumin. The limit of detection was 1 mg/kg whole
egg in the sample.
Table 8: Applications of Sandwich-ELISA for the detection of food
allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
| Almond (Protein)
Sensitivity: 100 ng/mL
(Almond Flour containing 21% Protein) |
Sesame Seed, Black Walnut, Macadamia, Pistachio, Brazil
Nut, Hazelnut, Cashew
Capture Antibody:
rabbit pAb
Secondary Antibody:
sheep pAb |
Samples: Cereals, Chocolate, Dairy Foods, Confectionary
Items
Limit of Detection:
1 mg/kg (Almond)
Recovery: 86-100% |
Hlywka et al. 2000 |
| Hazelnut (Protein)
Sensitivity:
1-600 ng/mL
|
Pumpkin Seed, Walnut, and Cashew (tolerable amounts of
10, 20, and 50%, respectively)
Capture Antibody:
rabbit pAb
Secondary Antibody:
sheep pAb |
Samples: Chocolates, Chocolate Products, Muesli
Limit of Detection:
2 mg/kg
Recovery: 67-132%
CV: <15% |
Holzhauser & Vieths 1999b |
| Peanut (Protein)
Sensitivity:
5-1000 ng/mL
|
Almond, Cashew
Capture Antibody:
rabbit pAb
Secondary Antibody:
same Ab, labelled |
Samples: Cookies, Chocolate Bars and Candy, Sate
Sauce
Limit of Detection:
0.1 mg/kg
Recovery: 35-75% |
Koppelman et al. 1996 |
| Soybean (Gly m Bd 30K)
Sensitivity:
10-500 ng/well
(2-200 ng/well for reduced and carboxymethylated allergen) |
No cross-reactivity to other soybean allergens
Capture Antibody:
mice mAb
Secondary Antibody:
mice mAb
(both raised against reduced and carboxymethylated allergen) |
Samples: Soy Milk, Tofu, Kori-Dofu, Yuba, Meat
Balls, Beef Croquettes, Fried Chicken, Fermented Soybean Products
Range of Detection:
140-700 mg/kg
CV: 4-17% |
Tsuji et al. 1993, 1995 |
| Egg White (Ovalbumin)
Sensitivity:
not available |
Portobello Mushroom, Basil Leaves (no cross-reactivity
to other selected pasta ingredients)
Capture Antibody:
goat pAb (anti-Egg White)
Secondary Antibody:
rabbit pAb (anti-Ovalbumin) |
Samples: Several Pastas
Limit of Detection:
1 mg/kg (Whole Egg) |
Hefle et al. 2001 |
| Substrate
/
Product
Antibody
(Enzyme-labelled
)
Human
Serum IgE
Analyte
(Inhibitor)
Immobilized
Antigen
Solid Phase Support |
 |
|
EAST-Inhibition of
Ovomucoid (Gal d 1)
|
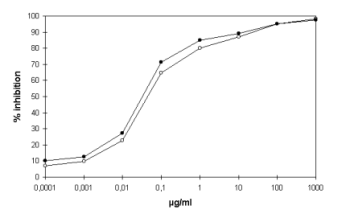
Figure 6: Principle of RAST or EAST-Inhibition |
Principle
RAST or EAST inhibition represent a kind of Competitive ELISA employing
human serum IgE antibodies. A solid phase bound antigen is involved which
binds specific human IgE (Figure 6). Standard or sample analytes inhibit
IgE binding to the solid phase bound antigen. An enzyme-labeled antibody
is used to detect the bound human IgE antibodies. The substrate-enzyme
reaction gives a colored product. The standard curve in Figure 6 shows
the inhibition of IgE-binding to the major hen's egg allergen ovomucoid
(self-inhibition compared to deglycosylated ovomucoid). |
Applications
RAST / EAST inhibition applications are seldom used to quantitate allergens
in foods (Table 10). One example is the detection of alpha-Lactalbumin
in baby food and food quality lactose (Frémont et al. 1996). The
standard curve gave a range of detection from 100 ng/mL to 10 µg/mL,
resulting in a limit of detection of 1 mg/kg in the samples.
The other applications shown in Table 10 were not used for the determination
of hidden allergens. The hazelnut RAST inhibition was used to compare the
performance with a Competitive ELISA format (Koppelman et al. 1999), while
the peanut RAST inhibition was used to compare the allergenic potential
of different peanut varieties (Koppelman et al. 2000).
The major drawback of RAST or EAST inhibition with respect to quantitation
is its reliance on non-standardized human sera whose amounts are often
limited. Furthermore, variable specificities of human IgE antibodies hinder
the use in a wider range of analytical laboratories. In addition commercial
solid-phases of food allergens can vary considerably in IgE-binding activities.
These limitations prevent commercial applications to quantitate food allergens
by RAST / EAST inhibition (Taylor & Nordlee 1995).
RAST/EAST inhibition has been applied for qualitative allergen detection
and for the assessment of allergenic potencies in a wide range of food
products, e.g:
-
Detection of codfish allergens in surimi, a Japanese food product imitating
shrimps, and pizza toppings by RAST inhibition (Helbling et al. 1992, Mata
et al. 1994).
-
IgE-binding potencies of hypoallergenic infant formulas in comparison to
cow's milk proteins (Oldaeus et al. 1991).
-
Assessment of the allergenic potencies of protein extracts from a wide
range of peanut containing food products such as peanut flour, roasted
peanuts, peanut butter, and hydrolyzed peanut protein (Nordlee et al. 1981),
or crude, neutralized, and refined peanut oil (Olszewskiet al. 1998) in
comparison to peanut protein extract.
-
The allergenic potencies of various soybean products such as raw soybeans,
sprouts, acid- hydrolyzed sauce, tofu, hydrolyzed vegetable protein, tempeh,
miso, and mold-hydrolyzed sauce were characterized by RAST inhibition (Herian
et al. 1993).
-
Characterization of heat and hydrolytic stability of hazelnut allergens
by EAST inhibition (Wigotzki et al. 2000 a, b).
Table 10: Applications of RAST or EAST-inhibition for the detection
of food allergens
| Food Allergen |
Cross-Reactivities |
Applications |
Reference |
Cow's Milk (alpha-Lactalbumin)
Sensitivity:
100 - 10000 ng/mL |
not available
Antisera:
human IgE |
Samples: Baby Food, Food Quality Lactose
Limit of Detection:
1 mg/kg |
Frémont et al. 1996 |
| Hazelnut (Protein)
Sensitivity:
30-1000 ng/mL
|
Walnut, Cashew, Pecan Nut, Pistachio
Antisera:
human IgE |
Limit of Detection:
6 mg/kg |
Koppelman et al. 1999 |
| Peanut (Protein)
Sensitivity:
approximately 50-300 ng/mL
|
not available
Antisera:
human IgE |
Samples: 13 Different Peanut Samples
Relative Allergenicity:
Comparison of 50%-Inhibition
CV: 10% |
Koppelman et al. 2000 |
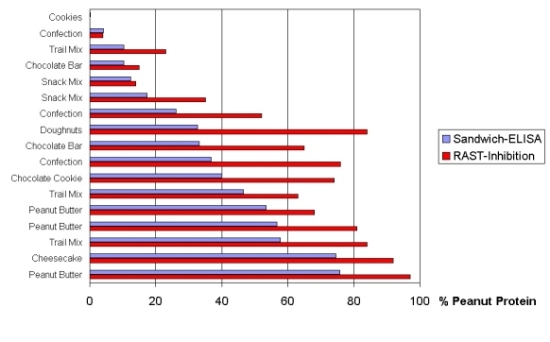
Figure 7 shows the results of the determination of peanut protein
by a Sandwich-ELISA (the violet bars) as compared to RAST-Inhibition (the
red bars) (Hefle et al. 1994). A significant overestimation of peanut content
by RAST-Inhibition was demonstrated in 15 of 17 different food samples.
The major cause of overestimation is probably a high degree of cross-reactivities
of the human IgE antibodies to other food ingredients than peanuts. A pooled
serum from about 10 patients was used in this study. It is most likely
that these patients had some concomitant IgE-sensitizations.
This example reflects the major disadvantage of RAST / EAST inhibition.
As mentioned above it is difficult to obtain standardized antisera. Human
sera are often limited. Furthermore every patient serum has a different
individual pattern of IgE-specificities.
Therefore RAST or EAST inhibition is seldom used for the determination
of allergens in foods, but it is an ideal tool for the characterization
of IgE-binding properties reflecting the allergenic potential of crude
protein extracts, purified food allergens, allergenic activities of different
varieties and various processed foods.
|
| Figure 7: ELISA versus RAST: Determination of Peanut Protein (data
from Hefle et al. 1994) |
ELISA
TEST KITS
Table 11 gives an overview of commercially available Test-Kits with sufficient
limits of detection for the determination of food allergens. To date there
are ELISA-Test-Kits available for egg, milk, peanut, and wheat.
It is obvious that Tests for many other important food allergens are
not available. For the screening and quality control of food products it
recommended to use standardized, evaluated Test-Kits to obtain reproducible
and precise results minimizing the risk of false negative and false positive
results, respectively.
Table 11: Commercially available ELISA Test Kits
|
Food Allergen
|
Limit of Detection
|
Trademark / Company |
| Egg, Milk, Peanut |
10 mg/kg |
Veratox / Neogen |
a) Peanut
b) Wheat Gluten |
a) 0.5 - 2 mg/kg
b) 20 / 200 mg/kg |
BioKits / Tepnel BioSystems
ELISA-TEK / ELISA Technologies |
a) Egg White (Ovalbumin)
b) Milk (beta-Lactoglobulin)
c) Peanut
d) Wheat (omega-Gliadin) |
a) 5 mg/kg
b) 5 mg/kg
c) 2.5 mg/kg
d) 5 mg/kg |
Ridascreen / R-Biopharm |
a) Milk (Caseins)
b) Milk (beta-Lactoglobulin)
c) Peanut
d) Wheat (omega-Gliadin) |
a) 25 mg/kg
b) 25 mg/kg
c) 0.5 - 2 mg/kg
d) 10 mg/kg |
Transia (Tepnel BioSystems, Diffchamb S.A.) |
FREQUENCY
OF HIDDEN FOOD ALLERGENS
Undeclared Allergens in Foods
Unfortunately, up to now, there are no systematic studies on the frequency
of hidden allergens in foods. There have been only very few investigations
which analyzed more than a few samples. These samples are most probably
food samples suspected to contain a certain allergen, meaning these studies
may not be representative for the investigated food products as a whole.
But nevertheless, the data listed in Table 12 indicate that a significant
number of foods contain undeclared allergens. 43% of 28 analyzed chocolates
and chocolate products and mueslis contained undeclared amounts of hazelnut
protein (Holzhauser & Vieths 1999b). In another study 58% of 26 similar
food samples contained undeclared hazelnut protein (Koppelman et al. 1999).
Undeclared peanut proteins were detected in 29% of 17 samples (Holzhauser
& Vieths 1999a). While Schäppi et al. (2001) detected undeclared
peanut proteins in 5 of 7 products (cereal bars, corn crackers, potato
snacks).
In a recent study 83 chocolates supposed to be free of almond and hazelnut
were analyzed (Scheibe et al. 2001). Almond was detected in 61% and hazelnut
in 72% of samples, respectively.
Table 12: Frequency of Hidden Allergens in Foods not Declared on
the Label
| Food Samples* |
Undeclared Allergen |
Percentage |
Reference |
| 28 Chocolates, Chocolate Products, Muesli |
Hazelnut |
43 % |
Holzhauser & Vieths 1999b |
| 17 Roasted Cashews, Chocolates, Nuts and Chocolate, Raisin
and Chocolate, Coconut Cookie, Amarettini, Cereal Bars |
Peanut |
29 % |
Holzhauser & Vieths 1999a |
| 26 Chocolate Spreads, Bars, and Cookies, Muesli Cookie,
Cake |
Hazelnut |
58 % |
Koppelman et al. 1999 |
| 83 Chocolates |
Almond
Hazelnut |
61 %
72 % |
Scheibe et al. 2001 |
* Please note: samples may not be representative for the
kind of foods investigated.
Foods Labeled as Being Free of Allergens
In contrast, food products labeled as being free of a certain allergen
contained significantly less frequently hidden allergens. But nevertheless,
again, a significant number of samples was contaminated with hidden allergens
(Table 13).
In the case of egg, 1.3% of 319 samples contained egg protein. Milk
proteins were detected in 2.3% of 838 samples, and wheat in 5.2% of 1583
samples. These results demonstrate the difficulty of producing "allergen
free" products.
It should be noted that the samples and detection methods were not
indicated. Therefore the majority of samples could be samples suspected
to contain the related allergen.
Table 13: Frequency of hidden allergens in foods labeled as being
free of the respective allergen
| Food Samples* |
Labeled as being free of |
Percentage |
Reference |
| 319 (not specified) |
Egg |
1.3 % |
Standing Committee for Foodstuffs 1997 |
| 838 (not specified) |
Milk |
2.3 % |
Standing Committee for Foodstuffs 1997 |
| 1583 (not specified) |
Wheat (Gluten) |
5.2 % |
Standing Committee for Foodstuffs 1997 |
* Please note: samples may include complain samples not be
representative for the kind of foods investigated.
CONCLUSIONS
At present immunoassays are the method of choice to determine hidden
food allergens. Suitable immunological methods for the detection of trace
amounts of allergens in foods are the rocket immunoelectropheresis, with
a sensitivity of less than 5 µg/mL; SDS/PAGE- and dot-immunoblot
applications, with sensitivites in the range of 30 to 200 ng/mL, and ELISA
methods with sensitivities of approximately 0.1 to 100 ng/mL. Immunodiffusion
techniques usually have an insufficient sensitivity, in the range of 10-20
µg/mL. In summary:
-
Immunoassays are specific, sensitive, and rapid methods (usually 2 to 4
hours) to detect and quantitate even trace amounts of allergens in food
products.
-
Standardized (commercial) ELISA-Test-Kits are available for egg, milk,
peanut, and wheat proteins only.
-
Test-Kits for soybean, hazelnut (and other tree nuts), sesame seed, celery,
fish and shellfish are not available at the moment.
-
Furthermore there is a need for reliable and cost-effective screening methods
which can rapidly detect minute amounts of food allergens.
REFERENCES
-
Ballmer-Weber BK, Vieths S, Lüttkopf D, Heuschmann P,
Wüthrich B (2000) Celery allergy confirmed by DBPCFC. A clinical
study in 32 subjects with a history of adverse reactions to celery rootJ
Allergy Clin Immunol 106:373-8
-
Besler (2001) Determination of allergens in foods[review]
Trends
Anal Chem 20:662-72
-
Blais BW, Phillippe LM (2000) A cloth-based enzyme immunoassay
for detection of peanut proteins in foods Food Agric Immunol 12:243-8
-
Blais BW, Phillippe L (2001) Detection of hazelnut proteins
in foods by enzyme immunoassay using egg yolk antibodies J Food
Protection 64:895-8
-
Bock SA, Anne Muñoz-Furlong A, Sampson HA (2001)
Fatalities
due to anaphylactic reactions to foods J Allergy Clin Immunol 107:191-3
-
Bousquet J, Björksten B, Bruijnzeel-Koomen CAFM, Hugget
A, Ortolani C, Warner JO, Smith M (1998) Scientific criteria and the
selection of allergenic foods for product labelling Allergy 53:3-21
-
Citizen Petition to The U.S. Department of Health and Human
Services Food and Drug Administration (2000) Allergenic SubstancesAttorney
General, New York State, Press Release [ http://www.oag.state.ny.us/press/2000/may/may26a_00.html
]
-
Codex Alimentarius Commission (1999) Food labelling -
complete texts
Joint FAO/WHO Food Standards Programme. FAO/WHO,
Rome
-
Deibel K, Trautman T, DeBoom T, Sveum WH, Dunaif G, Scott
VN, Bernard DT (1997) A comprehensive approach to reducing the risk
of allergens in foods J Food Protection 60:436-41
-
Denery-Papini S, Nicolas Y, Popineau Y (1999) Efficiency
and limitations of immunochemical assays for the testing of gluten-free
foods J Cereal Sci 30:121-31
-
European Commission (1998)
Reports on tasks for scientific
cooperation. The occurrence of severe food allergies in the EU European
Commission, Directorate-General III, Brussels
-
European Commission (2001)
Proposal for a DIRECTIVE OF
THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
amending Directive 2000/13/EC as regards indication
of the ingredients present in foodstuffs Commission of the European
Communities, COM(2001) 433 final, 2001/0199 (COD), Brussels, 06.09.2001
-
Gern JE, Yang E, Evrard HM, Sampson HA (1991) Allergic
reactions to milk-contaminated "nondairy" products N Engl J Med
324:976-9
-
Hansen TK, Bindslev-Jensen C (1992) Codfish allergy in
adults. Identification and diagnosis Allergy 47:610-7
-
Hefle SL, Bush RK, Yunginger JW, Chu FS (1994) A sandwich
enzyme-linked immunosorbent assay (ELISA) for the quantitation of selected
peanut proteins in foods J Food Protection 57(5):419-23
-
Hefle SL, Jeanniton E, Taylor SL (2001) Development of
a sandwich enzyme-linked immunosorbent assay for the detection of egg residues
in processed foods J Food Protection 64:1812-6
-
Helbling A, Lopez M, Lehrer SB (1992) Fish allergy: is
it a real problem with surimi-based products ? Int Arch Allergy
Immunol 99:452-5
-
Herian AM, Taylor SL, Bush RK (1993) Allergenic reactivity
of various soybean products as determined by RAST inhibition J Food
Science 58:385-8
-
Hlywka JJ, Hefle SL, Taylor SL (2000) A sandwich enzyme-linked
immunosorbent assay for the detection of almonds in foods J Food
Protection 63:252-7
-
Holzhauser T, Vieths S (1999a) Indirect competitive ELISA
for determination of traces of peanut (Arachis hypogaea) protein
in complex food matrices J Agric Food Chem 47:603-11
-
Holzhauser T, Vieths S (1999b) Quantitative Sandwich ELISA
for the determination of traces of hazelnut (Corylus avellana) protein
in complex food matrices J Agric Food Chem 47:4209-18
-
Holzhauser T, Wangorsch A, Vieths S (2000) Polymerase
chain reaction (PCR) for detection of potentially allergenic hazelnut residues
in complex food matrixes Eur Food Res Technol 211:360-5
-
Hourihane JO'B, Kilburn SA, Nordlee JA, Hefle SL, Taylor
SL, Warner JO (1997) An evaluation of the sensitivity of subjects with
peanut allergy to very low doses of peanut protein: a randomized, double-
blind, placebo-controlled food challenge study J Allergy Clin Immunol
100:596-600
-
Hugget AC, Hischenhuber C (1998) Food manufacturing initiatives
to protect the allergic consumer Allergy 53(Suppl.46):89-92
-
Keck-Gassenmeier B, Benet S, Rosa C, Hischenhuber C (1999)
Determination
of peanut traces in food by a commercially-available ELISA test Food
Agric Immunol 11:243-50
-
Koeppel E, Stadler M, Luethy J, Huebner P (1998) Detection
of wheat contamination in oats by polymerase chain reaction (PCR) and enzyme-linked
immunosorbent assay (ELISA) Zeitschrift fuer Lebensmittel-Untersuchung
und -Forschung A 206(6):399-403
-
Koppelman SJ, Knulst AC, Koers WJ, Penninks AH, Peppelman
H, Vlooswijk R, Pigmans I, van Duijn G, Hessing M (1999) Comparison
of different immunochemical methods for the detection and quantification
of hazelnut proteins in food products J Immunological Methods 229:107-20
-
Lipton CR, Dautlick JX, Grothaus GD, Hunst PL, Magin KM,
Mihaliak CA, Rubio FM, Stave JW (2000) Guidelines for the valication
and use of immunoassays for determination of introduced proteins in biotechnology
enhanced crops and derived food ingredients Food Agric Immunol 12:153-64
-
Malmheden Yman I, Eriksson A, Everitt G, Yman L, Karlsson
T (1994) Analysis of food proteins for verification of contamination
or mislabelling Food Agric Immunol 6:167-72
-
Mariager B, Solve M, Eriksen H, Brogen CH (1994) Bovine
beta-lactoglobulin in hypoallergenic and ordinary infant formulas measured
by an indirect competitive ELISA using monoclonal and polyclonal antibodies
Food
Agric Immunol 6:73-83
-
Mata E, Favier C, Moneret-Vautrin DA, Nicolas JP, Han-Ching
L, Gueant JL (1994) Surimi and native codfish contain a common allergen
identified as a 63-kDa protein Allergy 49:442-7
-
Miller JB (1978) Hidden food ingredients, chemical food
additives and incomplete food labels Ann Allergy 41:93-8
-
Mills C, Potts A, Plumb GW, Lambert N, Morgan MRA (1997)
Development
of a rapid dipstick immunoassay for the detection of peanut contamination
of food Food Agric Immunol 9:37-50
-
Morisset M, Moneret-Vautrin DA (2001)
Thresholds
of clinical reactivity to foods: milk, egg, peanut and sesame - Evaluation
by standardised placebo-controlled oral challenges Lecture, CICBAA/SHS
Symposium, September 20-21, Nancy, France (Alim' Inter Vol.7, Numero Special
- Janvier 2002)
-
Nordlee JA, Taylor SL, Jones RT, Yunginger JW (1981) Allergenicity
of various peanut products as determined by RAST inhibition J Allergy
Clin Immunol 68:376-82
-
Norgaard A, Bindslev-Jensen C (1992) Egg and milk allergy
in adults. Diagnosis and characterization Allergy 47:503-9
-
Oldaeus G, Bjorksten B, Einarsson R, Kjellman NIM (1991)
Antigenicity
and allergenicity of cow milk hydrolysates intended for infant feedingPediatr
Allergy Immunol 2:156-64
-
Olszewski A, Pons L, Moutete F, Aimone-Gastin I, Kanny G,
Moneret-Vautrin DA, Gueant JL (1998) Isolation and characterization
of proteic allergens in refined peanut oil Clin Exp Allergy 28:850-9
-
Sampson HA (1998) Fatal food-induced anaphylaxis Allergy
53 (Suppl 46):125-30
-
Schäppi GF, Konrad V, Imhof D, Etter R, Wüthrich
B (2001) Hidden peanut allergens detected in various foods: findings
and legal measures Allergy 56:1216-20
-
Scheibe B, Weiss W, Rueff F, Przybilla B (2001) Detection
of trace amounts of hidden allergens: hazelnut and almond proteins in chocolate
J
Chromatogr B 756:229-37
-
Standing Committee for Foodstuffs (1997) Report – Co-ordinated
programme for the official contral of foodstuffs for 1997 European
Commission, Brussels
-
Taylor SL, Hefle SL, Bindslev-Jensen C, Bock SA, Burks AW,
Christie L, Hill DJ, Host A, Hourihane JO’B, Lack G, Metcalfe DD, Moneret-Vautrin
DA, Vadas PA, Rance F, Skrypec DJ, Trautman TA, Malmheden Yman I, Zeiger
RS (2002) Factors affecting the determination of threshold doses for
allergenic foods: How much is too much? J Allergy Clin Immunol 109:24-30
-
Tsuji H, Bando N, Kimoto M, Okada N, Ogawa T (1993) Preparation
and application of monoclonal antibodies for a sancwich enzyme-linked immunosorbent
assay of the major soybean allergen, Gly m Bd 30K J Nutr Sci Vitaminol
39:389-97
-
Tsuji H, Okada N, Yamanishi R, Bando N, Kimoto M, Ogawa T
(1995) Measurement of Gly m Bd 30K, a major soybean allergen, in soybean
products by a sandwich enzyme-linked immunosorbent assay Biosci
Biotechnol Biochem 59:150-1
-
Vadas P, Wai Y, Burks W, Perelman B (2001) Detection of
peanut allergens in breast milk of lactating women JAMA 285:1746-8
-
Wigotzki M, Schubert S, Steinhart H, Paschke A (2000a) Effects
of in vitro digestion on the IgE-binding activity of proteins from hazelnuts
(Corylus
avellana) Internet Symposium on Food Allergens 2:1-8
-
Wigotzki M, Steinhart H, Paschke A (2000b) Influence of
varieties, storage and heat treatment on IgE-binding proteins in hazelnuts
(Corylus
avellana) Food Agric Immunol 12:217-29
-
Wigotzki M, Steinhart H, Paschke A (2001) Determination
of the allergenicity of various hazelnut products by immunoblotting and
enzyme allergosorbent test inhibition J Chromatogr B 756:239-48
-
Wüthrich B (2000) Lethal or life-threatening allergic
reactions to food Invest Allergol Clin Immunol 10:59-65
-
JM Yeung, PG Collins (1996) Enzyme immunoassay for determination
of peanut proteins in food products J AOAC Int 79:1411
-
Yeung JM, Applebaum RS, Hildwine R (2000) Criteria to
determine food allergen priority J Food Protection 63:982-6
[Summary]
[Abbreviations]
copyright © 2002 by matthias besler -
ONLINE PUBLISHER
home: www.food-allergens.de